The Role of Hypoxia in Wound Healing: A Double-Edged Signal in Tissue Repair
Technical Note
Introduction
Wound healing is a complex and highly orchestrated biological process involving the interaction of various cell types, signaling molecules, and extracellular matrix components. Oxygen tension plays a critical regulatory role in this process. Although oxygen is essential for energy metabolism and cell survival, hypoxia, a condition of reduced oxygen availability, acts as a central modulator in wound healing. Paradoxically, hypoxia can both promote and impair wound repair depending on its duration, severity, and the cellular context in which it occurs. Understanding this dual role is critical for developing therapeutic strategies that modulate oxygen tension to enhance healing outcomes, especially in chronic wounds and tissue engineering applications.
Hypoxia in the Wound Microenvironment
Immediately after tissue injury, the local vasculature is disrupted, resulting in a precipitous drop in oxygen tension at the wound site. In healthy skin, oxygen levels range from 30–50 mmHg (4-7% oxygen), whereas wound tissues may drop below 10 mmHg (1% oxygen) due to impaired perfusion and increased metabolic demand. The initial hypoxic state is not merely a consequence of injury but acts as a physiological signal that initiates cellular responses aimed at tissue restoration.
Hypoxia activates key transcriptional programs, primarily through hypoxia-inducible factors (HIFs), which upregulate genes involved in angiogenesis, cell survival, glucose metabolism, and extracellular matrix remodeling. The expression of HIF-1α, the most widely studied member of the HIF family, is rapidly induced under hypoxic conditions and stabilized by the inhibition of prolyl hydroxylase domain enzymes (PHDs), which normally mark HIF-1α for proteasomal degradation in the presence of oxygen.
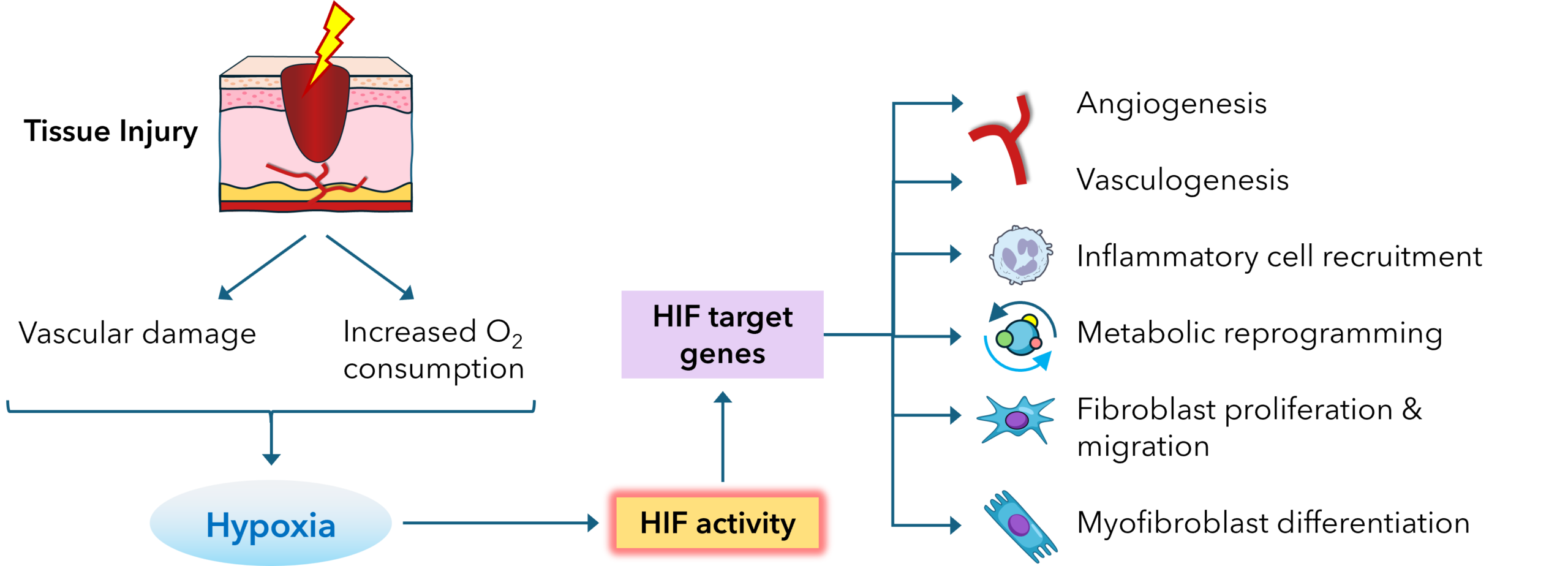
Effects of Hypoxia on Cellular Functions During Wound Healing
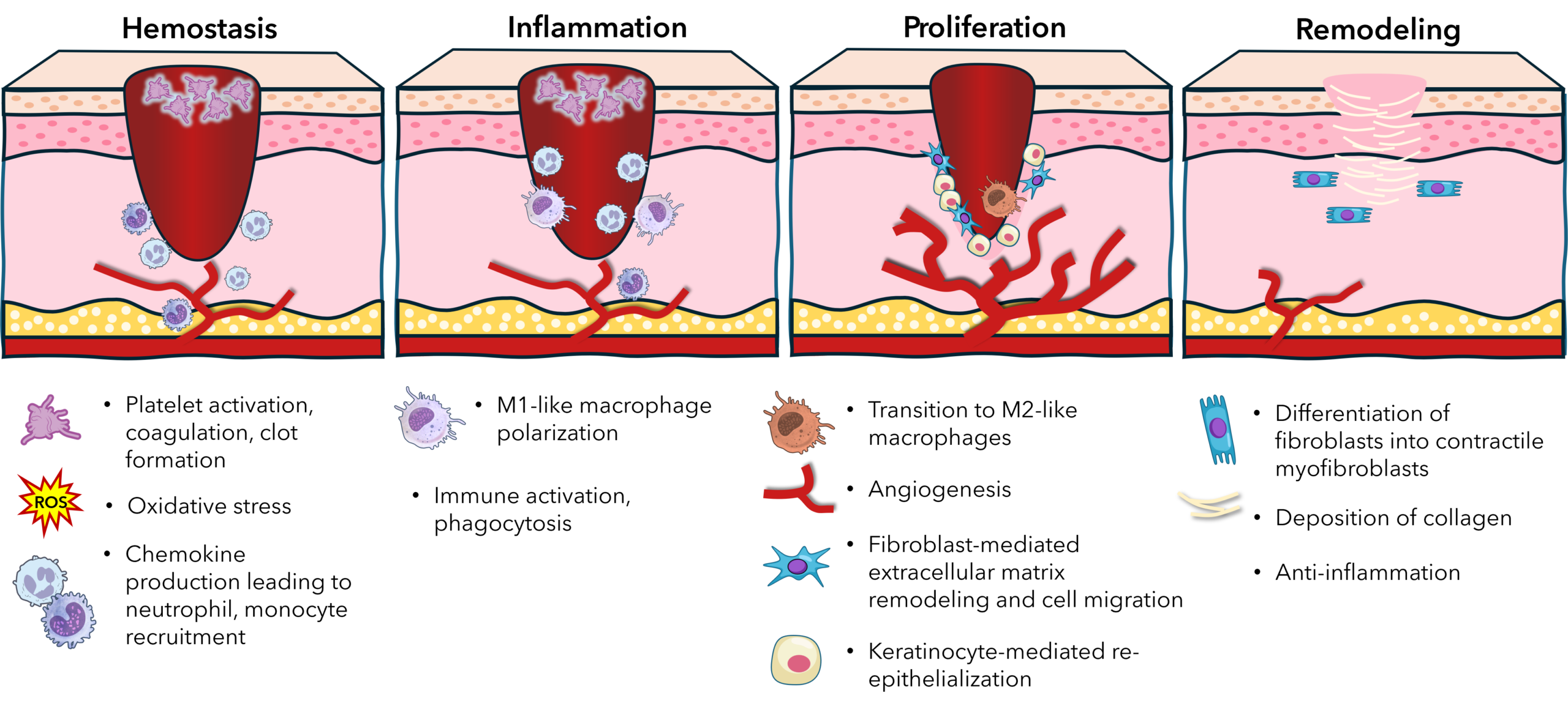
Wound healing can be divided into four overlapping stages: hemostasis, inflammation, proliferation, and remodeling. Hypoxia influences multiple cell types across each of these phases.
1. Hemostasis Phase
When a wound forms, local oxygen levels drop sharply due to disrupted blood flow; this hypoxia can enhance platelet activation and aggregation, partly via increased thromboxane A2 and hypoxia-induced changes in endothelial cell function, helping stabilize the clot. However, severe or systemic hypoxia (as in poor perfusion or vascular disease) can impair fibrin formation and prolong bleeding time, potentially compromising initial clot stability.
2. Inflammatory Phase
In the early inflammatory phase, hypoxia enhances the recruitment of neutrophils and monocytes by stimulating the production of chemokines such as CXCL12 and MCP-1. Macrophages are highly responsive to hypoxic cues. Under
low oxygen, macrophages shift to a pro-inflammatory (M1-like) phenotype, producing reactive oxygen species (ROS), TNF-α, and IL-1β, which are essential for pathogen clearance and debris removal. However, sustained hypoxia may impair macrophage polarization toward the reparative (M2-like) phenotype, delaying the resolution of inflammation in chronic wounds.
3. Proliferation and Angiogenesis
Hypoxia plays a pivotal role in promoting angiogenesis, which is vital for restoring oxygen supply to the wound bed. HIF-1α upregulates vascular endothelial growth factor (VEGF), angiopoietin-2, and platelet-derived growth factor-B (PDGF-B), driving endothelial cell proliferation, migration, and neovascularization. Fibroblasts also respond to hypoxia by enhancing collagen synthesis and secreting matrix metalloproteinases (MMPs), which facilitate extracellular matrix
remodeling and cell migration.
In parallel, keratinocytes at the wound edge respond to hypoxia by activating migratory programs that enable re-epithelialization. HIF-1α promotes β1-integrin expression and facilitates epidermal stem cell activation, supporting epithelial closure. However, excessive or prolonged hypoxia may induce keratinocyte apoptosis or senescence, particularly under ischemic conditions.
4. Fibroblast Function and Myofibroblast Differentiation
Fibroblasts, central to the proliferative and remodeling phases, are sensitive to oxygen tension. Under moderate hypoxia (1–5% O2), fibroblasts increase proliferation and secretion of ECM proteins, including type I and III collagen and fibronectin. Importantly, hypoxia also promotes the differentiation of fibroblasts into contractile myofibroblasts via TGF-β1 signaling, facilitating wound contraction.
However, excessive or chronic hypoxia may induce fibroblast dysfunction, reduce matrix deposition, and increase susceptibility to oxidative stress. This can result in tissue fibrosis or chronic non-healing wounds, depending on the balance of profibrotic and matrix-degrading signals.
Hypoxia in Chronic Wounds: A Pathological State
While acute hypoxia serves as a beneficial signal for repair, chronic hypoxia—as seen in diabetic ulcers, pressure sores, and venous leg ulcers—contributes to impaired healing. In diabetic wounds, for example, microvascular dysfunction and impaired angiogenic response result in persistent ischemia. Hyperglycemia also interferes with HIF-1α signaling, diminishing VEGF expression and endothelial cell recruitment. This leads to poor granulation tissue formation, defective re-epithelialization, and a heightened risk of infection.
Moreover, chronic hypoxia impairs the metabolic flexibility of cells. Mitochondrial dysfunction, elevated ROS levels, and cellular senescence are all commonly observed in chronic wounds. Fibroblasts in such wounds often adopt a senescent phenotype, characterized by decreased proliferative capacity and the secretion of pro-inflammatory cytokines—a state known as the senescence-associated secretory phenotype (SASP).
Therapeutic Modulation of Hypoxia
Given the nuanced role of hypoxia in wound healing, therapeutic interventions aim to either alleviate detrimental chronic hypoxia or enhance beneficial hypoxic signaling. Strategies include:
- Hyperbaric Oxygen Therapy (HBOT): Used clinically to increase oxygen delivery to hypoxic tissues, particularly in chronic and diabetic wounds. HBOT promotes angiogenesis and collagen synthesis, although patient response can be variable.
- Prolyl Hydroxylase Inhibitors (PHIs): Small molecules that stabilize HIF-1α even in normoxic conditions, boosting endogenous VEGF and EPO production. These are under investigation for promoting wound vascularization and regeneration.
- Oxygen-Releasing Biomaterials: Engineered wound dressings that generate or deliver oxygen in a controlled manner (e.g., calcium peroxide-loaded hydrogels) can sustain physiological oxygen levels in the wound bed, balancing hypoxia and reoxygenation.
- Cell-Based Therapies: Mesenchymal stem cells (MSCs) and exosomes preconditioned under hypoxia demonstrate improved survival, paracrine signaling, and regenerative capacity when transplanted into wounds.
Conclusion
Hypoxia is both a signal and a stressor in the wound healing cascade. While transient hypoxia activates key regenerative pathways through HIF signaling, sustained or pathological hypoxia impairs cellular function, angiogenesis, and matrix remodeling. The fine balance between these effects highlights the need for context-specific therapeutic modulation of oxygen availability. Advances in oxygen-delivery technologies, HIF-targeted agents, and hypoxia-responsive biomaterials offer promising strategies to harness the beneficial aspects of hypoxia while mitigating its detrimental consequences in impaired wound healing.
References
- Han X, Ju LS, Irudayaraj J. Oxygenated Wound Dressings for Hypoxia Mitigation and Enhanced Wound Healing. Mol Pharm. 2023 Jul 3;20(7):3338-3355. doi: 10.1021/acs.molpharmaceut.3c00352. Epub 2023 Jun 20. PMID: 37338289; PMCID: PMC10324602..
- Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells. 2014 Sep;37(9):637-43. doi: 10.14348/molcells.2014.0150. Epub 2014 Jun 24. PMID: 24957212; PMCID: PMC4179131.
- Steiner CA, Cartwright IM, Taylor CT, Colgan SP. Hypoxia-inducible factor as a bridge between healthy barrier function, wound healing, and fibrosis. Am J Physiol Cell Physiol. 2022 Sep 1;323(3):C866-C878. doi: 10.1152/ajpcell.00227.2022. Epub 2022 Aug 1. PMID: 35912990; PMCID: PMC9467472.
- Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008 Sep;34(9):1159-69. doi: 10.1111/j.1524-4725.2008.34254.x. Epub 2008 May 29. PMID: 18513296.
Download PDF Version
Featured Content
Join our eNewsletter
Stay up-to-date with our latest technical notes, announcements, and new products.