Differential Regulation of Autophagy Under Hypoxic, Physioxic, and Normoxic Conditions
Technical Note
Introduction
Autophagy, a highly conserved catabolic process, is critical for maintaining cellular homeostasis by degrading and recycling damaged organelles and macromolecules. Oxygen availability plays a pivotal role in regulating autophagy, and recent studies have highlighted that the oxygen levels used in conventional cell culture experiments may not accurately reflect in vivo physiological conditions. This technical note aims to summarize and compare the regulation of autophagy under hypoxic (<2% O2), physioxic (2-6% O2), and normoxic (21% O2) conditions, emphasizing how each oxygen environment differentially influences autophagic activity.
Normoxia (Atmospheric Oxygen ~21%)
Traditionally, in vitro experiments are conducted under atmospheric oxygen levels (~21%), often referred to as “normoxia.” However, this is significantly higher than oxygen tensions found in most tissues in vivo. At 21% O2, cells experience an artificially hyperoxic environment, which can lead to increased oxidative stress and altered metabolic pathways.
Studies have shown that cells cultured under normoxic conditions may exhibit baseline autophagic activity, but this state does not truly reflect physiological autophagy regulation. The presence of higher ROS (reactive oxygen species) levels due to oxidative stress may activate certain autophagic pathways, but it may also initiate cell damage and senescence, thereby confounding experimental interpretations. For example, in mesenchymal stem cells (MSCs) and neural progenitor cells (NPCs), culturing under normoxia has been associated with premature senescence and reduced self-renewal capacity, partially mediated through dysregulated autophagy.
Physioxia (Physiological Oxygen ~2-6%)
Physioxia refers to the oxygen tension that more closely mirrors in vivo conditions, which varies by tissue type but generally falls between 2% and 6%. For example, oxygen levels in bone marrow, brain, and adipose tissue typically lie within this range. Recent research has emphasized the importance of using physioxic conditions in vitro to better replicate cellular behavior seen in vivo.
Autophagy under physioxia is modulated in a more controlled and balanced manner compared to normoxia. In this environment, basal autophagic activity is maintained without excessive ROS production, thereby supporting cellular homeostasis. Physiological oxygen levels stabilize HIF-1α (hypoxia-inducible factor 1-alpha) to a moderate extent, which can upregulate key autophagy-related genes such as BNIP3 and NIX. These genes promote mitophagy (selective autophagy of mitochondria), ensuring quality control and metabolic adaptation.
Several studies have demonstrated that physioxia preserves stemness and enhances regenerative potential in various stem cell populations, partly due to well-regulated autophagic flux. In human adipose-derived stem cells (ASCs), for instance, 2% O2 culture conditions led to increased proliferation and reduced senescence, with a concomitant rise in autophagic markers including LC3-II and Beclin-1. Similarly, neural progenitor cells cultured under 3% O2 exhibit improved mitochondrial function and enhanced antioxidant capacity, which are supported by a balanced autophagic response.
Hypoxia (<2% O2)
Hypoxic conditions, defined as oxygen levels below 2% typically found in tumor microenvironments and ischemia, act as a significant stressor for cells and robustly activate autophagy as a survival mechanism. Under hypoxia, HIF-1α is strongly stabilized and transactivates a host of target genes, including BNIP3, NIX, and REDD1, which modulate autophagy through both mTOR-dependent and mTOR independent pathways.
The activation of autophagy in hypoxia involves several key mechanisms:
- HIF-1α/BNIP3 Axis: Upregulation of BNIP3 and NIX disrupts the Beclin-1/Bcl-2 interaction, freeing Beclin-1 to initiate autophagosome formation.
- AMPK Activation: Cellular energy stress under hypoxia leads to AMP-activated protein kinase (AMPK) activation, which inhibits mTOR (a negative regulator of autophagy), thereby promoting autophagic flux.
- ROS Signaling: Hypoxia-induced ROS can further stimulate autophagy, although excessive ROS may also trigger apoptosis if not tightly regulated.
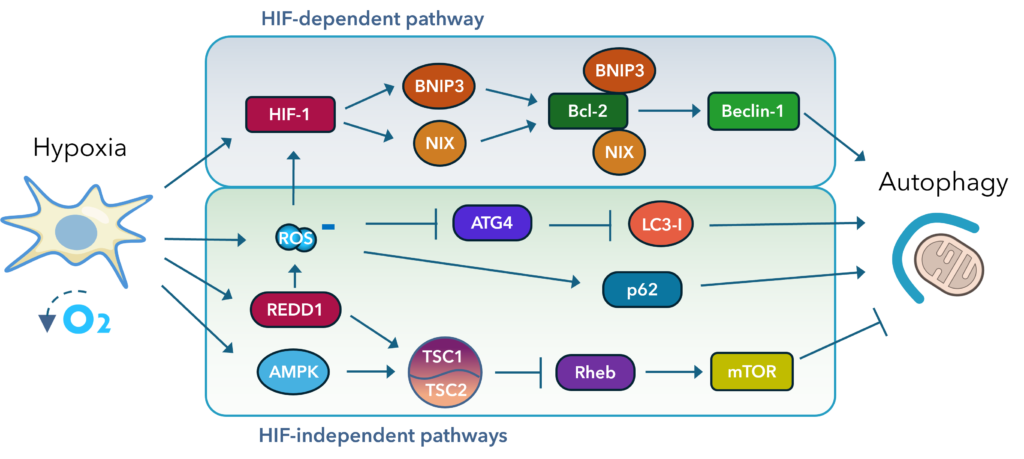
Fig. 1. Hypoxia drives multiple pathways leading to increased autophagy.
While autophagy activation under hypoxia can be protective by maintaining energy homeostasis and removing damaged organelles, chronic or severe hypoxia may shift this balance toward cell death pathways, including autophagic cell death or apoptosis. In cancer biology, hypoxia-induced autophagy supports tumor cell survival in nutrient-poor environments, contributing to therapy resistance and aggressive phenotypes.
Comparative Overview
| Oxygen Condition | O2 Range | HIF-1α Stabilization | ROS Levels | Autophagy Status | Functional Impact |
| Normoxia | ~21% | Low | High | Basal to mild | Potential for oxidative damage, altered metabolism |
| Physioxia | 2-6% | Moderate | Low-moderate | Balanced | Maintains homeostasis, enhances stemness and metabolic fitness |
| Hypoxia | <2% | High | High | Strongly induced | Survival mechanism, potential for autophagic cell death |
Conclusion
Oxygen tension is a critical factor influencing autophagy, with distinct regulatory mechanisms operating under normoxic, physioxic, and hypoxic conditions. Normoxic conditions, although commonly used in cell culture, impose hyperoxic stress that can distort autophagy-related observations. Physioxia provides a more physiologically accurate model, supporting balanced autophagic flux and improved cell function. Hypoxia triggers a potent autophagic response to counteract cellular stress, but prolonged exposure may lead to deleterious effects. Recognizing these differences is essential for accurately interpreting autophagy-related studies and for optimizing experimental models to reflect true physiological or pathological states.
References
- Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009 Jul;16(7):939-46. doi: 10.1038/cdd.2009.16. Epub 2009 Feb 20. PMID: 19229244; PMCID: PMC2768230.
- Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007 Sep;27(17):6229-42. doi: 10.1128/MCB.02246-06. Epub 2007 Jun 18. PMID: 17576813; PMCID: PMC1952167.
- Mazure NM, Pouysségur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010 Apr;22(2):177-80. doi: 10.1016/j.ceb.2009.11.015. Epub 2009 Dec 21. PMID: 20022734.
- Li J, Gong SH, He YL, Cao Y, Chen Y, Huang GH, Wang YF, Zhao M, Cheng X, Zhou YZ, Zhao T, Zhao YQ, Fan M, Wu HT, Zhu LL, Wu LY. Autophagy Is Essential for Neural Stem Cell Proliferation Promoted by Hypoxia. Stem Cells. 2023 Jan 30;41(1):77-92. doi: 10.1093/stmcls/sxac076. PMID: 36208284.
- Rakesh R, PriyaDharshini LC, Sakthivel KM, Rasmi RR. Role and regulation of autophagy in cancer. Biochim Biophys Acta Mol Basis Dis. 2022 Jul 1;1868(7):166400. doi: 10.1016/j.bbadis.2022.166400. Epub 2022 Mar 25. PMID: 35341960.
Download PDF Version
Featured Content
Join our eNewsletter
Stay up-to-date with our latest technical notes, announcements, and new products.




