Hypoxia-Preconditioned Exosomes as a New Therapeutic Approach
Technical Note
Introduction
Exosomes are small extracellular vesicles (EVs), typically ranging from 30 to 150 nm in diameter, secreted by nearly all cell types into body fluids, including blood, urine, and saliva. These vesicles carry various bioactive molecules, such as proteins, lipids, and RNA, which play crucial roles in intercellular communication. Due to their ability to transport molecular cargo across biological barriers, exosomes are gaining increasing attention for their therapeutic potential, particularly in the treatment of diseases such as cancer, neurodegenerative disorders, and cardiovascular
conditions.
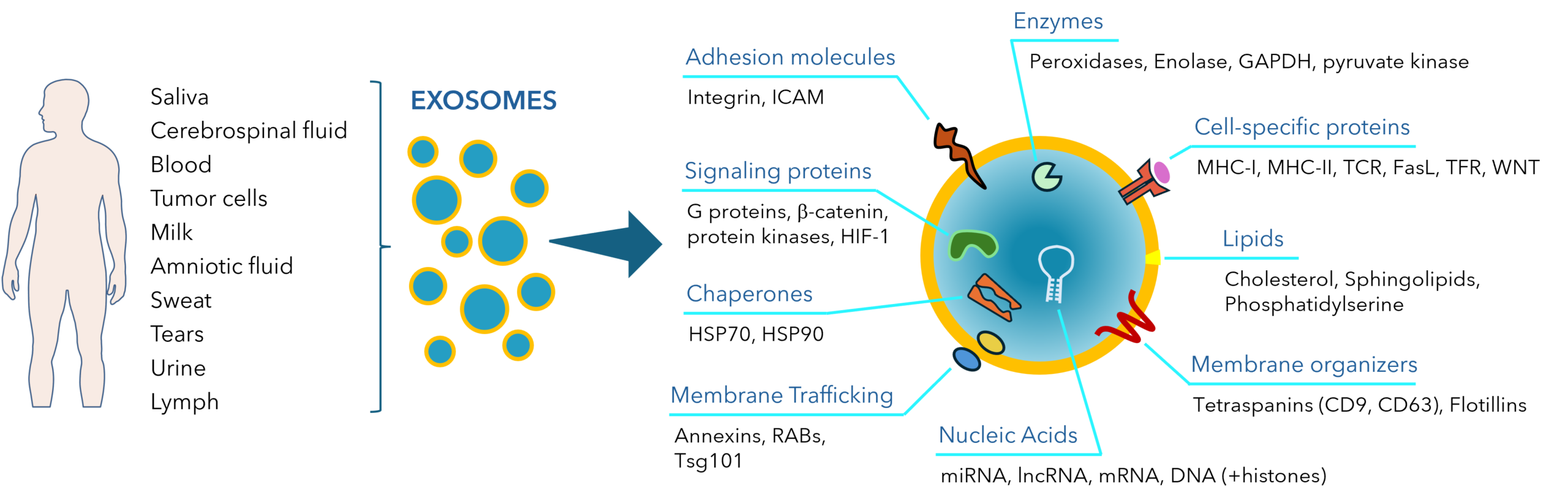
Fig. 1. Sources and typical components of human exosomes.
One of the emerging strategies to enhance the therapeutic efficacy of exosomes involves hypoxic preconditioning of the cells from which exosomes are derived. Hypoxia, a condition characterized by low oxygen levels, triggers cellular responses that can enhance exosome production and their bioactive cargo. Hypoxia-preconditioned exosomes (HPEs) have shown promise as novel therapeutics due to their ability to mimic the beneficial effects of hypoxia on cellular functions while overcoming some of the limitations associated with traditional hypoxic therapies. This technical note explores the current state of research on hypoxia-preconditioned exosomes and their potential applications in medicine.
Hypoxia and Cellular Responses
Hypoxia is a common feature of many pathological conditions, including ischemic heart disease, stroke, and cancer. Cells exposed to low oxygen levels adapt by activating a range of signaling pathways, most notably through the hypoxia-inducible factor (HIF) family of transcription factors. These factors promote the expression of genes involved in cell survival, angiogenesis, metabolic adaptation, and inflammation. Hypoxic conditions also lead to alterations in cellular metabolism, increased autophagy, and changes in gene expression, all of which can enhance tissue repair and regeneration.
While the physiological response to hypoxia is beneficial under certain circumstances, prolonged exposure to low oxygen can lead to tissue damage and pathological conditions. This has led researchers to explore strategies that exploit the beneficial effects of hypoxia without inducing harmful consequences. One such strategy is the use of hypoxia-preconditioned exosomes.
Mechanism of Hypoxia-Preconditioned Exosomes
Exosomes are secreted by cells in response to various stimuli, including hypoxia. Under hypoxic conditions, cells undergo significant changes in the composition of exosome cargo. Studies have shown that hypoxia leads to increased secretion of exosomes containing specific miRNAs, proteins, and other molecules that contribute to cell survival, angiogenesis, and tissue repair. These bioactive molecules can be transferred to recipient cells, influencing their behavior and promoting regenerative processes.
The molecular basis for the enhanced therapeutic potential of HPEs lies in the changes in their cargo composition following exposure to hypoxia. For instance, hypoxia increases the secretion of exosomes containing miRNAs that regulate gene expression, including those involved in vascular remodeling, cell proliferation, and apoptosis. Additionally, proteins such as heat shock proteins (HSPs), which are induced under stress conditions, are often found in higher concentrations in exosomes derived from hypoxic cells. These proteins have been shown to protect cells from oxidative stress and promote tissue repair.
The key signaling pathways involved in the production of hypoxia-preconditioned exosomes include the HIF pathway, the PI3K/Akt pathway, and the MAPK/ERK pathway. HIF-1α, the most studied member of the HIF family, plays a central role in regulating the expression of genes involved in exosome production and cargo composition. It has been shown that hypoxic conditions lead to the upregulation of proteins such as HIF-1α, which in turn activates various cellular processes, including the production and release of exosomes.
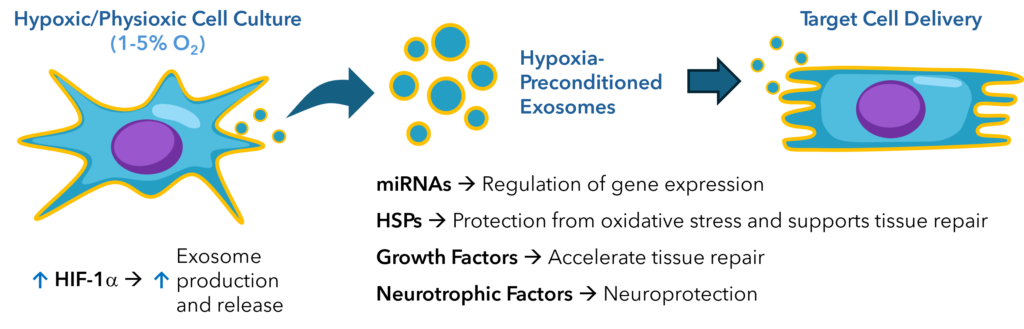
Fig. 2. HIF-1α drives the production of hypoxia-preconditioned exosomes that have enhanced functionality, improving cell survival, angiogenesis, and tissue repair.
Therapeutic Applications of Hypoxia-Preconditioned Exosomes
Recent studies have investigated the impact of low oxygen conditions on IVF outcomes:
- Cardiovascular Disease: Hypoxia-preconditioned exosomes have shown potential in the treatment of cardiovascular diseases, particularly in the context of ischemic heart disease. In animal models, HPEs have been demonstrated to promote angiogenesis, improve myocardial function, and protect against ischemic damage. The cargo of these exosomes, including pro-angiogenic miRNAs and proteins, facilitates vascular regeneration and enhances tissue repair. By delivering these bioactive molecules to ischemic tissues, HPEs can mimic the effects of ischemic preconditioning, a phenomenon where brief episodes of ischemia protect the heart from subsequent prolonged ischemic injury.
- Cancer Therapy: Exosomes derived from hypoxic tumor cells have been implicated in promoting tumor growth, metastasis, and resistance to therapy. However, engineered exosomes or those derived from healthy or preconditioned cells have shown promise as therapeutic agents. HPEs can be loaded with anti-cancer agents or miRNAs that target oncogenic signaling pathways. Moreover, HPEs can enhance the delivery of therapeutic agents to tumor cells, as they are capable of crossing biological barriers, including the blood-brain barrier in the case of neurological tumors. In addition, their natural ability to target tumor cells makes them attractive candidates for targeted cancer therapy.
- Neurodegenerative Diseases: Hypoxia is often observed in the brains of patients with neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. HPEs have demonstrated neuroprotective properties in preclinical models of these conditions. The exosomes can carry neurotrophic factors, anti-inflammatory molecules, and miRNAs that regulate neurogenesis and neuronal survival. Additionally, the ability of HPEs to cross the blood-brain barrier makes them particularly useful for treating neurological conditions where traditional drug delivery methods are often limited.
- Wound Healing and Tissue Regeneration: HPEs are also being explored for their potential in tissue regeneration and wound healing. The exosomal cargo, which may include growth factors and anti-inflammatory molecules, can accelerate tissue repair and reduce fibrosis in injured tissues. By enhancing cell migration, proliferation, and differentiation, HPEs can contribute to faster and more efficient healing of wounds, especially in cases of chronic wounds or injuries associated with poor vascularization.
Challenges and Future Directions
Despite the promising potential of hypoxia-preconditioned exosomes, several challenges must be addressed before their widespread clinical application. One major challenge is the standardization of exosome isolation and characterization methods. The heterogeneous nature of exosomes complicates their purification, and variations in the cargo content can lead to inconsistent therapeutic outcomes. Additionally, the potential immunogenicity of exosomes, especially when derived from non-autologous sources, remains a concern.
Another challenge lies in scaling up exosome production. While laboratory-scale production of exosomes is feasible, the production of large quantities required for clinical applications presents significant technical and economic hurdles. Advances in bioreactor systems and optimization of exosome isolation techniques are critical to overcoming these challenges.
Lastly, the long-term safety and efficacy of HPEs need to be carefully evaluated in clinical trials. The potential for off-target effects, as well as the risk of unintended consequences arising from the complex biological activity of exosomes, requires thorough investigation.
Conclusion
Hypoxia-preconditioned exosomes represent a promising class of therapeutic agents with the potential to treat a wide range of diseases, including cardiovascular disorders, cancer, neurodegenerative diseases, and chronic wounds. Their ability to carry bioactive molecules that promote tissue repair, angiogenesis, and cell survival makes them ideal candidates for regenerative medicine. However, challenges related to standardization, production scalability, and safety must be addressed before their clinical translation can be realized. With continued research and development, hypoxia-preconditioned exosomes may become a powerful tool in modern therapeutic strategies.
References
- Jafari R, Rahbarghazi R, Ahmadi M, Hassanpour M, Rezaie J. Hypoxic exosomes orchestrate tumorigenesis: molecular mechanisms and therapeutic implications. J Transl Med. 2020 Dec 10;18(1):474. doi: 10.1186/s12967-020-02662-9. PMID: 33302971; PMCID: PMC7731629.
- Toghiani R, Azimian Zavareh V, Najafi H, Mirian M, Azarpira N, Abolmaali SS, Varshosaz J, Tamaddon AM. Hypoxia- preconditioned WJ-MSC spheroid-derived exosomes delivering miR-210 for renal cell restoration in hypoxia- reoxygenation injury. Stem Cell Res Ther. 2024 Jul 30;15(1):240. doi: 10.1186/s13287-024-03845-7. PMID: 39080774; PMCID: PMC11289969.
- Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Zhou Z, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020 Feb;103:196-212. doi: 10.1016/j.actbio.2019.12.020. Epub 2019 Dec 17. PMID: 31857259.
- Fan MH, Zhang XZ, Jiang YL, Pi JK, Zhang JY, Zhang YQ, Xing F, Xie HQ. Exosomes from hypoxic urine-derived stem cells facilitate healing of diabetic wound by targeting SERPINE1 through miR-486-5p. Biomaterials. 2025 Mar;314:122893. doi: 10.1016/j.biomaterials.2024.122893. Epub 2024 Oct 15. PMID: 39418849.
- Shi Y, Wang S, Wang K, Yang R, Liu D, Liao H, Qi Y, Qiu K, Hu Y, Wen H, Xu K. Relieving Macrophage Dysfunction by Inhibiting SREBP2 Activity: A Hypoxic Mesenchymal Stem Cells-Derived Exosomes Loaded Multifunctional Hydrogel for Accelerated Diabetic Wound Healing. Small. 2024 Jun;20(25):e2309276. doi: 10.1002/smll.202309276. Epub 2024 Jan 21. PMID: 38247194.
- Yang H, Tu Z, Yang D, Hu M, Zhou L, Li Q, Yu B, Hou S. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci Lett. 2022 Jan 19;769:136389. doi: 10.1016/j.neulet.2021.136389. Epub 2021 Dec 8. PMID: 34896256.
- Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z. HIF-1α overexpression in mesenchymal stem cell- derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther. 2020 Aug 28;11(1):373. doi: 10.1186/s13287-020-01881-7. PMID: 32859268; PMCID: PMC7455909.
Download PDF Version
Featured Content
Join our eNewsletter
Stay up-to-date with our latest technical notes, announcements, and new products.




