Lessons from Vioxx: The Importance of Physioxia in Drug Toxicity Screening
Technical Note
Introduction
Drug discovery and development critically depend on the accurate evaluation of candidate compounds for both efficacy and safety. A key component of this process is drug toxicity screening, which helps identify potential adverse effects before clinical trials. However, for decades, toxicity studies have predominantly been performed under normoxic conditions; ambient atmospheric oxygen of approximately 21%. This level of oxygen does not reflect the physiologic oxygen tensions that cells experience in vivo, where most tissues are exposed to much lower oxygen levels, typically ranging from 1–8%, a state often termed physioxia. Growing recognition of this discrepancy has revealed that physiologically relevant oxygen levels are essential for improving the predictive power of toxicity assays, reducing late-stage drug failures, and ensuring safer therapies.
This technical note highlights the importance of physioxic conditions in drug toxicity screening, explores mechanistic insights into why oxygen tension affects toxicity outcomes, and examines the historical case of Vioxx (rofecoxib) as an example of the consequences of inadequate predictive models.
Physiologic Oxygen and Cellular Metabolism
Cells in the human body exist in environments far below atmospheric oxygen. For example, brain tissue oxygen tensions range from 1–5%, the liver averages 4–8%, and bone marrow is typically around 1–6% [1,2]. These oxygen ranges are tightly linked to cellular metabolism, mitochondrial function, and gene expression. A central mediator of the cellular response to oxygen is hypoxia-inducible factor (HIF), which regulates a wide array of genes involved in metabolism, angiogenesis, and stress responses [3].
When cells are cultured in normoxia, they are effectively hyperoxic compared to their in vivo environment. This artificial condition alters baseline physiology, including reactive oxygen species (ROS) production, mitochondrial respiration, and drug metabolism. Consequently, toxicity outcomes measured under normoxic culture may misrepresent how a compound behaves in the human body [4].
Oxygen Tension and Drug Toxicity Mechanisms
Toxicity can arise from multiple mechanisms, including mitochondrial dysfunction, oxidative stress, and metabolic activation of drugs into reactive intermediates. Oxygen levels influence all of these processes:
- Mitochondrial Function: Mitochondria are the central hub of both energy production and drug-induced injury. At physioxia, mitochondrial respiration is tuned to oxygen availability, whereas normoxia exaggerates oxygen consumption and ROS production, potentially masking or amplifying toxicity [5].
- Oxidative Stress: Many drugs cause toxicity by generating oxidative stress. In normoxia, elevated ROS may artificially increase the observed toxicity of a drug, leading to false positives. Conversely, some compounds may appear safe under high oxygen but prove toxic under physiologic oxygen, where subtle redox imbalances more accurately reflect tissue vulnerability [6].
- Metabolic Enzyme Activity: Cytochrome P450 enzymes, critical in drug metabolism, are sensitive to oxygen tension. Culture at physioxia preserves more physiologically relevant enzyme activity, influencing drug bioactivation and detoxification pathways [7].
These mechanisms underscore that oxygen is not a neutral parameter in toxicity testing. Instead, it is a critical determinant of drug–cell interactions.
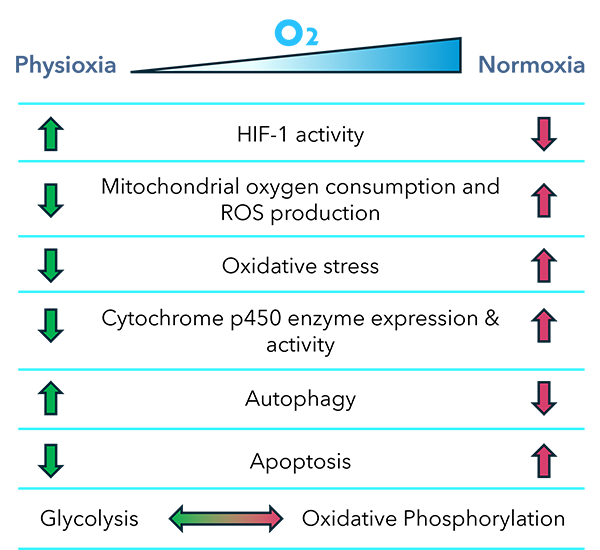
Fig. 1. Oxygen-tuned processes potentially affecting drug toxicity.
Historical Relevance: The Case of Vioxx
The story of Vioxx (rofecoxib), a selective cyclooxygenase-2 (COX-2) inhibitor, illustrates the consequences of incomplete toxicity prediction. Approved by the FDA in 1999, Vioxx was widely prescribed for arthritis and pain management. While initial trials suggested a favorable safety profile compared to nonselective NSAIDs, post-marketing surveillance revealed an increased risk of cardiovascular events, including heart attacks and strokes [8]. By 2004, Merck voluntarily withdrew Vioxx from the market, after it was estimated to have contributed to tens of thousands of excess cardiovascular deaths worldwide [9,10].
Several factors contributed to the failure to predict these adverse effects, but a critical issue was the reliance on models that did not fully recapitulate the physiological environment. Preclinical toxicity studies largely focused on gastrointestinal safety and failed to adequately capture cardiovascular risk. It is now understood that COX-2 inhibition disrupts the delicate balance between prostacyclin and thromboxane, tipping the scales toward vasoconstriction and thrombosis [11].
Importantly, vascular biology is highly oxygen-sensitive: endothelial and smooth muscle cells function within low-oxygen microenvironments that regulate prostaglandin synthesis, nitric oxide signaling, and platelet activation [12]. Had more physiologically relevant oxygen conditions been employed in toxicity studies, subtle effects on vascular function may have been detected earlier. Although we cannot directly attribute Vioxx’s failure to the absence of physioxic culture, the case underscores the broader need for models that more faithfully reproduce human physiology to anticipate off-target toxicity.
Modern Approaches: Incorporating Physioxia in Drug Screening
Advances in cell culture technology now enable researchers to better mimic physiologic oxygen conditions in vitro. Modular hypoxia chambers, oxygen-controlled incubators, and microfluidic organ-on-a-chip systems allow precise regulation of oxygen tension, enabling drug screening under physioxic conditions that mirror in vivo tissue environments [13]. Learn more about Embrient’s Modular Incubator Chamber.
Key benefits of physioxic drug screening include:
- Improved Predictive Accuracy: By reducing the mismatch between in vitro and in vivo oxygen levels, drug toxicity assays are more likely to reflect clinical outcomes.
- Enhanced Reproducibility: Controlled oxygen environments reduce variability introduced by oxidative stress artifacts in normoxic culture.
- Tissue-Specific Modeling: Different oxygen tensions can be tailored to model the microenvironments of specific organs, improving detection of organ-specific toxicities.
- Reduced Late-Stage Failures: With more accurate early screening, fewer drugs will fail in costly Phase III trials due to unforeseen toxicities.
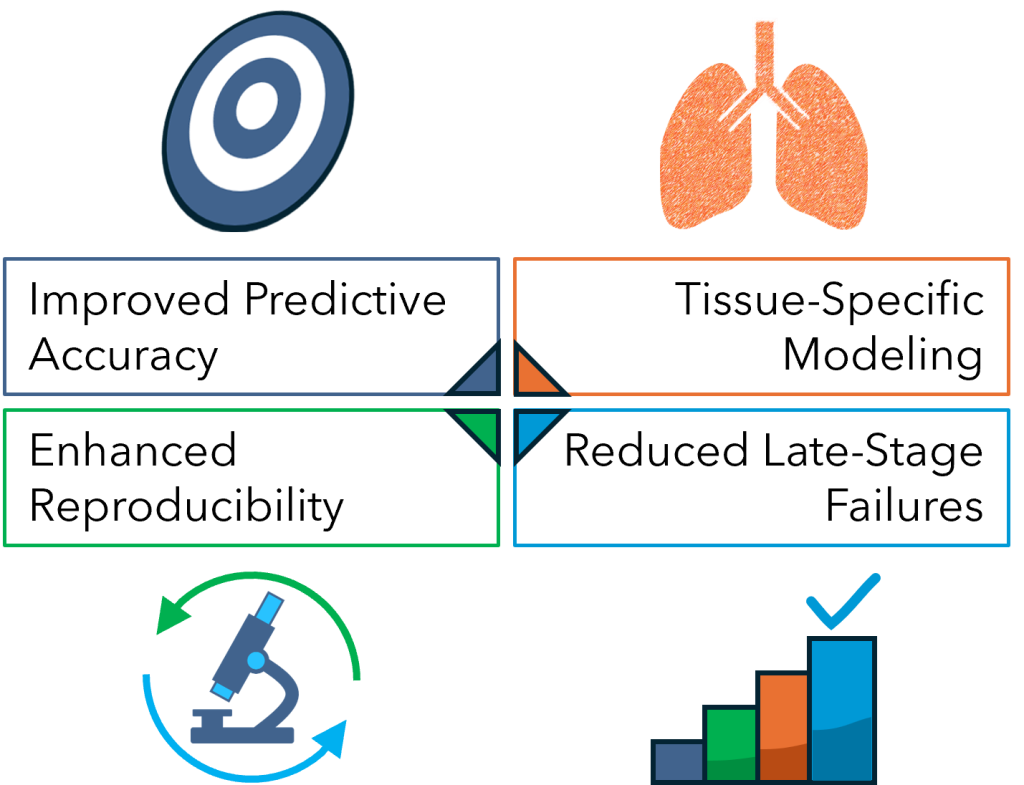
Fig. 2. Key benefits of physioxic drug screening.
Future Directions & Conclusion
The integration of physioxic conditions into drug discovery represents a paradigm shift. As regulators and industry stakeholders increasingly recognize the limitations of traditional models, physiologically relevant oxygen control may become a standard requirement for preclinical studies. Incorporating physioxia alongside other innovations, such as 3D organoid cultures and microphysiological systems, promises to significantly improve the translational power of toxicity screening.
The lessons from Vioxx remind us that even small physiological mismatches in preclinical testing can have profound real-world consequences. By embracing physioxia in drug toxicity screening, researchers and pharmaceutical companies can reduce risk, improve patient safety, and accelerate the development of effective therapeutics.
References
- Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small GTPases. 2011;2(4):207-210.
- Brahimi-Horn MC, Pouysségur J. Oxygen, a source of life and stress. FEBS Lett. 2007;581(19):3582-3591.
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365(6):537-547.
- Keeley TP, Mann GE. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol Rev. 2019;99(1):161-234.
- Zhdanov AV, et al. Monitoring of cell bioenergetics in vitro for assessment of drug safety. Pharmacol Ther. 2014;141(2):167-177.
- Halliwell B. Oxidative stress and cell proliferation. Free Radic Res Commun. 1990;9(1):1-10.
- Papandreou I, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187-197.
- Bombardier C, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520-1528.
- Bresalier RS, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092-1102.
- Graham DJ, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective NSAIDs: nested case-control study. Lancet. 2005;365:475-481.
- FitzGerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709-1711.
- Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. Physiol Rev. 2006;86(2):689-744.
- Park KM, Gerecht S. Hypoxia-inducible hydrogels. Nat Commun. 2014;5:4075.
Download PDF Version
Featured Content
Join our eNewsletter
Stay up-to-date with our latest technical notes, announcements, and new products.




